Making of Vaccines against Human Chorionic Gonadotrophin for Control of Fertility of Women without Impairment of Ovulation and Menstrual Regularity
Talwar GP, Gupta CJ, Nand NK, Thapa R and Mehta M
DOI10.21767/2476-1974.100004
Talwar GP*, Gupta JC, Nand KN, Thapa R and Mehta M
Talwar Research Foundation, New Delhi-110068, India
- *Corresponding Author:
- Talwar GP
Director Research, Talwar research Foundation
New Delhi-110068, India
Tel: +91-011-65022405
E-mail: gptalwar@gmail.com
Received date: January 11, 2016; Accepted date: February 17, 2016; Published date: February 22, 2016
Citation: Talwar GP, et al. Making of Vaccines against Human Chorionic Gonadotrophin for Control of Fertility of Women without Impairment of Ovulation and Menstrual Regularity. Reproductiv Immunol Open Acc. 2016, 1:4. doi: 10.4172/2476-1974.100004
Copyright: © 2016, Talwar GP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Reviewed briefly is the development of a unique vaccine against hCG, which prevents unwanted pregnancy without blocking ovulation and disturbing menstrual regularity. A recombinant version of this vaccine, amenable to largescale production by industry, in both DNA and protein form has been made. Priming with DNA followed by protein improves further its immunogenicity. Mycobacterium indicus pranii (MiP), a cultivable nonpathogenic mycobacteria has been used in the recombinant vaccine as a potent adjuvant. MiP was developed as an immuno-therapeutic vaccine against leprosy. It is approved by the Drugs Controller General of India and USFDA, and has many additional applications in treatment of tuberculosis, curing of ugly ano-genital warts and in prevention and treatment of some cancers.
Keywords
Recombinant vaccine; DNA priming; Potent adjuvant; Mycobacterium indicus pranii (MiP)
Introduction
Reproduction is made possible by suppression of the immune response which would normally be raised to the foetus carrying foreign genes of father, besides that of the mother. The objective of this article is not to discuss the considerable work that has been done on this issue, but to project how the immune system can be mobilized to prevent unwanted pregnancy without impairment of ovulation and menstrual regularity. The target of the potential fertility control vaccine is the human chorionic gonadotrophin (hCG). hCG is not secreted by any organ and is not normally present in circulation of the non-pregnant healthy female, the rationale on which diagnosis of pregnancy is based by detection of hCG in blood or urine for occurrence of pregnancy. hCG emerges soon after fertilization of the egg as a product of the early embryo. This was reported initially by Bob (Robert) Edwards, who eventually was honored by a Nobel prize [1].
The fact that hCG plays a crucial role in the implantation of the embryo onto the endometrium was demonstrated by John Hearn, who reported that marmoset embryos exposed to antihCG immunoglobins fail to implant, whereas the same embryos exposed to normal immunoglobins implant perfectly [2]. Thus our choice of hCG as a target for the eventual birth control vaccine in 1970s is justified by the above quoted work reported in the literature many years after we published our first paper on an anti-hCG vaccine developed by us [3].
hCG is a hormone composed of two subunits: alpha and beta. The alpha subunit of hCG is common to three other pituitary hormones, TSH, FSH and LH, the beta subunit in each case imparts the individual hormonal properties. It was thus logical for us to employ the beta subunit of hCG for the vaccine. The beta subunit of hCG is however not immunogenic in women. She makes enormous quantities of hCG during pregnancy and her immune system is fully tolerant to it. To make it immunogenic, we linked it with a carrier tetanus toxoid (TT), available at cheap rates in unlimited amounts from industrial sources.
An additional benefit of using TT as a carrier was that in case antibodies were also generated to the carrier, these will accord protection to the woman against tetanus. In those years a large number of deaths due to tetanus used to take place in India following delivery occurring in the field or at home, under aseptic conditions.
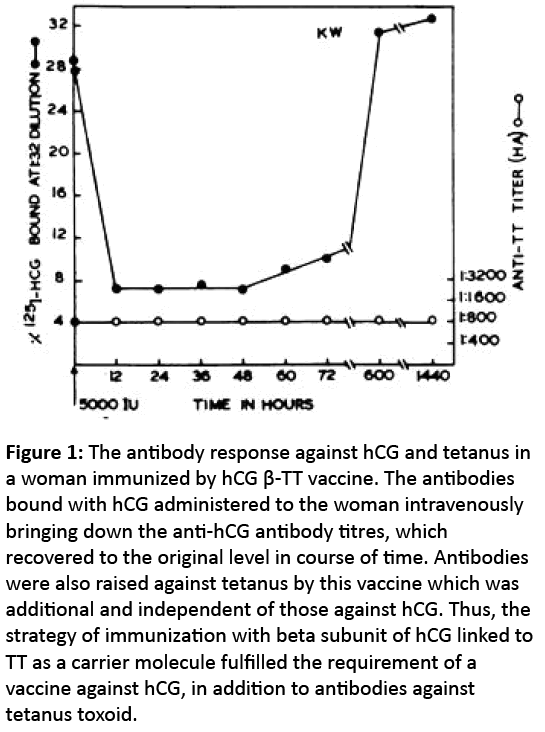
Ability of the antibodies generated by the hCGβ-TT vaccine to neutralize hCG bioactivity was tested by administration of a load dose of 5000 IU of hCG to a woman immunized by the vaccine. The vaccine generated in addition antibodies protective against tetanus [3] (Figure 1).
Figure 1: The antibody response against hCG and tetanus in a woman immunized by hCG β-TT vaccine. The antibodies bound with hCG administered to the woman intravenously bringing down the anti-hCG antibody titres, which recovered to the original level in course of time. Antibodies were also raised against tetanus by this vaccine which was additional and independent of those against hCG. Thus, the strategy of immunization with beta subunit of hCG linked to TT as a carrier molecule fulfilled the requirement of a vaccine against hCG, in addition to antibodies against tetanus toxoid.
Enhancement of Immunogenicity
Though hCG β-TT generated anti-hCG antibodies in all four women immunized with it, the antibody titres were not sufficiently high to counteract against the high amounts of hCG encountered in early pregnancy. An improved vaccine was made by associating non-covalently hCG beta with alpha subunit of ovine LH to create a hetero-species dimer (HSD) which was linked to TT. HSD-TT induced higher antibody response than hCG β-TT vaccine as shown in Table 1.
| Animal immunized | Immunogen* | hCG binding capacity Mean ± S.E.M. (pg) (I) | hCG neutralization potency Mean ± S.E.M. (pg) (B) | B/I x100 |
| Rats (n =6) | β hCG-TT/CHB | 27.1 ± 1.7 | 17.1 ± 1.2 | 63 ± 1.5 |
| Rats (n=6) | HSD-TT/CHB | 32.5 ± 1.4 | 26.1 ± 0.8 | 80 ± 2.3 |
| Bonnet monkeys (n=5) | β hCG-TT/CHB | 22.2 ± 2.3 | 10.1 ± 1.8 | 44 ± 3.7 |
| Bonnet monkeys (n=5) | HSD-TT/CHB | 21.4 ± 1.9 | 14.0 ± 1.4 | 65 ± 1.9 |
Table 1: hCG neutralization potency of anti-βhCG and anti-HSD antisera generated in rats and bonnet monkeys, a sub-human primate species.*Rats received three injections of 10 μg gonadotropin equivalent adsorbed on alum at monthly intervals. SPLPS (200 μg) as adjuvant was included in the first injection only. Bleeds were tested 1 week after the last immunization. Bonnet monkeys (Macaca radiata) were given three injections of 50 μg gonadotropin equivalent adsorbed on alum at monthly intervals. SPLPS (1 mg) was included as adjuvant in the first injection only. Bleeds were tested 2 weeks after the last immunization [4].
Does Anti-hCG Vaccine Prevent Pregnancy?
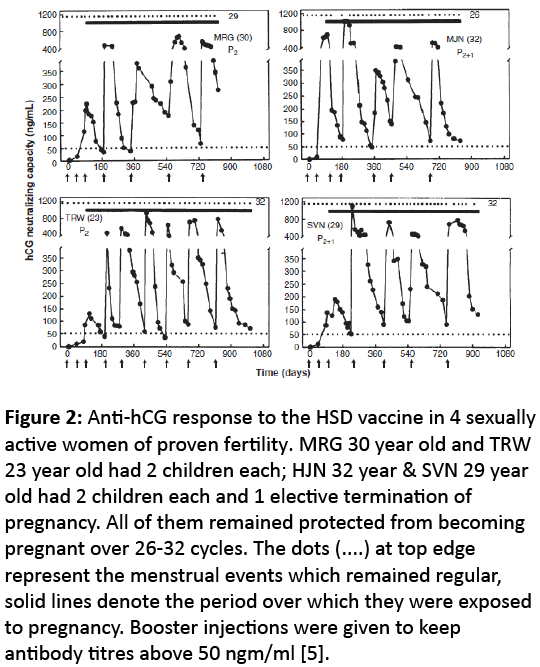
After due Regulatory and Ethics committees’ approval, Phase II efficacy trials were conducted in 148 sexually active women of proven fertility. A putative threshold of 50 ng/ml bioeffective anti-hCG titres was set for testing whether at these titres; it prevents pregnancy (Figure 2).
Figure 2: Anti-hCG response to the HSD vaccine in 4 sexually active women of proven fertility. MRG 30 year old and TRW 23 year old had 2 children each; HJN 32 year & SVN 29 year old had 2 children each and 1 elective termination of pregnancy. All of them remained protected from becoming pregnant over 26-32 cycles. The dots (....) at top edge represent the menstrual events which remained regular, solid lines denote the period over which they were exposed to pregnancy. Booster injections were given to keep antibody titres above 50 ngm/ml [5].
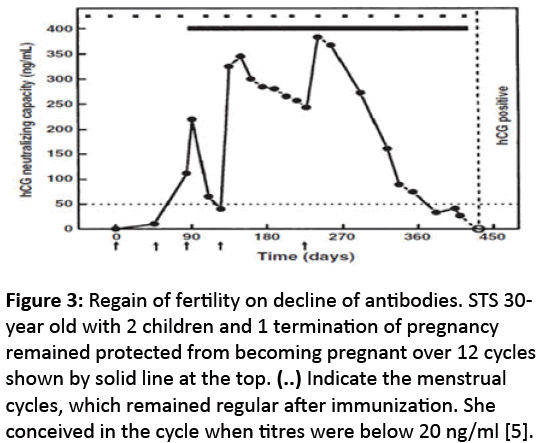
Although every woman immunized with HSD-TT vaccine made antibodies against hCG, 110 women had titres above 50 ng/ml for duration of three months or longer. IUD was removed in these women to expose them to pregnancy. Only 1 pregnancy occurred in 1224 of observation cycles indicating a high efficacy of anti-hCG antibodies to prevent pregnancy [5]. All women kept ovulating normally and continued to have regular menstrual cycles. The block of pregnancy was reversible. Fertility was regained on antibodies declining below 30 ngm/ml (Figure 3).
Figure 3: Regain of fertility on decline of antibodies. STS 30- year old with 2 children and 1 termination of pregnancy remained protected from becoming pregnant over 12 cycles shown by solid line at the top. (..) Indicate the menstrual cycles, which remained regular after immunization. She conceived in the cycle when titres were below 20 ng/ml [5].
The antibody response induced by the vaccine was reversible. The children born to previously immunized women were normal in their developmental landmarks and cognitive abilities as compared to their siblings [6].
Development of a Recombinant AntihCG Vaccine Amenable to Large Scale Industrial Production
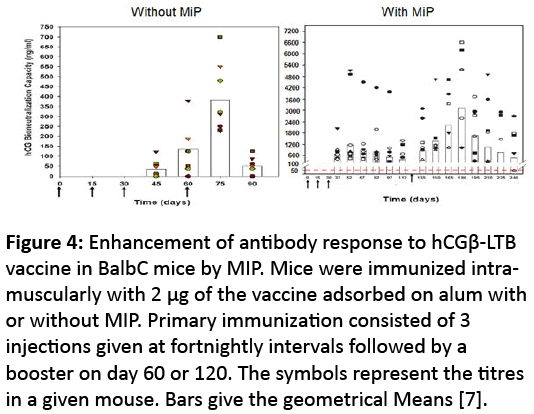
A recombinant hCGβ-LTB vaccine was made, which adsorbed on alum, along with Mycobacterium indicus pranii (MIP) used as adjuvant induced high anti-hCG titres in 100% of Balb c mice [7]. The vaccine was also immunogenic in every mouse of three other genetic strains of mice [8]. Figure 4a and 4b indicate the potent adjuvanticity of MIP.
Figure 4: Enhancement of antibody response to hCGβ-LTB vaccine in BalbC mice by MIP. Mice were immunized intramuscularly with 2 μg of the vaccine adsorbed on alum with or without MIP. Primary immunization consisted of 3 injections given at fortnightly intervals followed by a booster on day 60 or 120. The symbols represent the titres in a given mouse. Bars give the geometrical Means [7].
What is MIP?
Mycobacterium indicus pranii is a non-pathogenic mycobacteria originally developed by us as an immunotherapeutic and immuno-prophylatic vaccine against leprosy [9]. It was initially coded as M.w. Its ancestry and gene sequence has been determined. As no mycobacterium of this constitution existed in the World Data Bank, it has been named as Mycobacterium indicus pranii [10,11]. MIP shares antigens with both M. leprae and M. tuberculosis. Figure 5 is an electron micrograph of this bacillus.
Besides its properties of invigorating strongly cellular and humoral immune response, MiP as adjunct to drugs, expedites bacterial clearance in both multibacillary leprosy patients and category II, difficult to treat tuberculosis patients [12]. It brings about a dramatic recovery of ugly ano-genital warts [13]. Mycobacterium indicus pranii has received the approval of the Drugs Controller General of India and also of USFDA. It is licensed to Cadilla Pharma, and is available to public.
Further Enhancement of Antibody response to the Recombinant hCGβ- LTB Vaccine
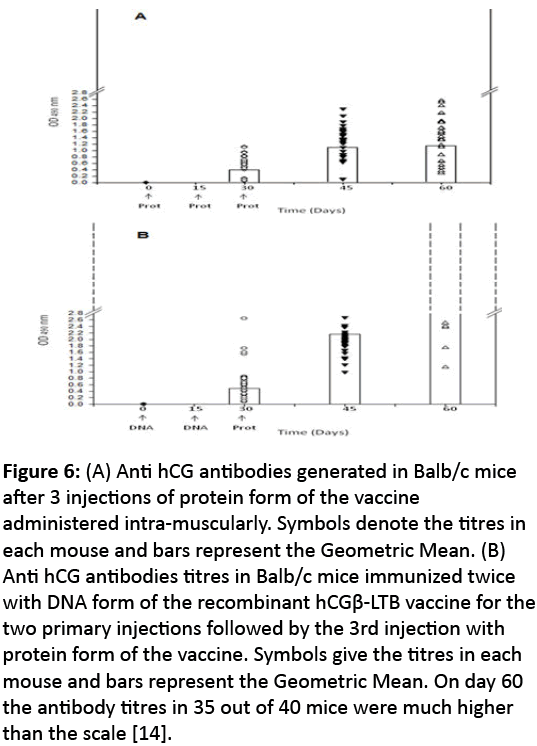
Recombinant vaccines can be made in both DNA and Protein versions. We employed eukaryotic plasmid VR1020 (DJ) for making the DNA version of the hCGβ-LTB vaccine. Plasmid VR1020 (DJ) is modified version of VR1020 (Vical Inc., San Diego, CA, USA) in which human CpG motifs are incorporated in the plasmid backbone for better adjuvanticity. Our immunization protocol demands initially 3 primary immunizations with the vaccine. While priming thrice with proteinic version of the vaccine achieved fairly high titres employing MiP as adjuvant, priming twice with the DNA version of the vaccine at fortnightly interval followed by the 3rd primary injection of the protein version, generated higher antibody response (Figure 6) than priming thrice with only the proteinic vaccine [14]. It may be stated that DNA vaccine is cheaper to make and is thermostable at room temperature thus requiring no cold chain.
Figure 6: (A) Anti hCG antibodies generated in Balb/c mice after 3 injections of protein form of the vaccine administered intra-muscularly. Symbols denote the titres in each mouse and bars represent the Geometric Mean. (B) Anti hCG antibodies titres in Balb/c mice immunized twice with DNA form of the recombinant hCGβ-LTB vaccine for the two primary injections followed by the 3rd injection with protein form of the vaccine. Symbols give the titres in each mouse and bars represent the Geometric Mean. On day 60 the antibody titres in 35 out of 40 mice were much higher than the scale [14].
Present Status of the Anti-hCG Vaccine
The recombinant hCGβ-LTB vaccine has received the approval of RCGM, the National Review Committee on Genetic Manipulation. It has undergone extensive toxicology studies in 2 species of rodents and in marmosets, a sub-human primate species. The vaccine is fully safe and devoid of any side effects and toxicity. Technology has been transferred to a Company, which will make available the vaccine made under GMP conditions for clinical trials to be conducted under the auspices of the Indian Council of Medical Research after due Ethical and Regulatory approvals.
Summary and Concluding Comments
A lot of research is in progress to develop vaccines for control of fertility. Vaccines against antigens on Spermatozoa and Zona pellucida of egg are being made. Amongst the hormones, vaccines against LHRH are effective for control of estrus of dogs and fertility of wild animals [15,16]. Against hCG, the only vaccine which has reached the stage of Phase II efficacy trials, is the one devised by us [5]. A recombinant vaccine amenable to industrial production has received the approval of RCGM and completed toxicology studies. It is expected to go back for clinical trials in the near future. An alternate vaccine based on using the C-terminal peptide of hCGβ demanded the use of a strong oily adjuvant [17]. Besides side-effects, it generated antibodies of lower affinity than the Km of hCG for binding to its receptors. Trials on the vaccine were abandoned in Sweden due to adverse reactions.
References
- Fishel SB, Edwards RG, Evans CJ (1984) Human chorionic gonadotropin secreted by pre-implantation embryo cultured in vitro. Science 223: 816-818.
- Hearn JP, Gidley-Baird AA, Hodges JK, Summers PM, Webley GE (1988) Embryonic signals during the pre-implantation period in primates. J Reprod Fertil 36: 49-58.
- Talwar GP, Sharma NC, Dubey SK, Salahuddin M, Das C, et al. (1976) Isoimmunisation against human chorionic gonadotropin with conjugates of processed ß-subunit of the hormone and tetanus toxoid. Proc Natl Acad Sci USA 73: 218-222.
- Talwar GP, Singh O, Rao LV (1988) An improved immunogen for anti-hCG vaccine reactive with conformation native to the hormone without cross-reaction with human follicle stimulating hormone and human thyroid stimulating hormone. J Reprod Immunol 14: 203-212.
- Talwar GP, Singh O, Pal R, Chatterjee N, Sahai P, et al. (1994) A vaccine that prevents pregnancy in women. Proc Natl Acad Sci USA 91: 8532-8536.
- Singh M, Das SK, Suri S, Singh O, Talwar GP (1998) Regain of fertility and normality of progeny born during below protective threshold antibody titres in women immunized with HSD-hCG vaccine. Am J Reprod. Immunol 39: 195-198.
- Puruswani S, Talwar GP (2011) Development of a highly immunogenic recombinant candidate vaccine against human chorionic gonadotropin. Vaccine 29: 2341-2348.
- Puruswani S, Talwar GP, Vohra R, Pal R, Panda AK, et al. (2011) Mycobacterium indicus pranii is a potent immunomodulator for a recombinant vaccine against human chorionic gonadotropin. J Reprod Immunol 91: 24-30.
- Talwar GP (1999) An immunotherapeutic vaccine for multibacillary leprosy. Intl Rev Immunol 18: 229-249.
- Talwar GP, Ahmed N, Saini V (2008) The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): a suggestion. Infect Genet Evol 8: 100-101.
- Saini V, Raghuvanshi S, Talwar GP, Ahmed N, Khurana JP, et al. (2009) Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS One 4: e6263.
- Patel N, Deshpande MM, Shah M (2002) Effect of an immunomodulatorcontaining Mycobacterium won sputum conversion in pulmonary tuberculosis. J Indian Med Assoc 100: 191-193.
- Gupta S, Malhotra AK, Verma KK, Sharma VK (2008) Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: an open label pilot study. J Eur Acad Dermatol Venereol 22: 1089-1093.
- Nand KN, Gupta JC, Panda AK, Jain SK, Talwar GP (2015) Priming with DNA enhances considerably the immunogenicity of hCGß-LTB vaccine. Am J Reprod Immunol 74: 302-308.
- Miller LA, Talwar GP, Killlian GJ (2006) Contraceptive effect of a recombinant GnRH vaccine in adult female pigs. In: Timm, R.M., O’Brien, J.M. (Eds.), Proc. 2 2nd Vertebr. Pest Conf. Univ. of Calif., Davis. USA, pp. 106-109.
- Talwar GP, Gupta SK, Singh V, Sahal D, Iyer KSN, et al. (1985) Bioeffective monoclonal antibody against the decapetide gonadotropin-releasing hormone: Reacting determinant and action on ovulation and estrus suppression. Proc Natl Acad Sci 82: 1228-1231.
- Steven VC, Cinaver B, Powell JE, Lee SE, Koh S (1981) Preparation and formulation of a hCG antifertility vaccine. Selection of adjuvant and vehicle. Am J Reprod Immunol Microbiol 6: 315-321.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences