NF-kB Regulation in T-cells in Pregnancy is Mediated via Fas/FasL Interactions: The Signal for which is Derived from Exosomes Present in Maternal Plasma
Sharon A McCracken, Katrina A Hadfield, Vanessa M Yenson, Gaayathri Ariyakumar, Kelly J McKelvey, Narelle Woodland, Anthony W Ashton and Jonathan M Morris.
DOI10.21767/2476-1974.100008
Sharon A McCracken1*, Katrina A Hadfield1, Vanessa M Yenson1, Gaayathri Ariyakumar1, Kelly J McKelvey1, Narelle Woodland2, Anthony W Ashton1 and Jonathan M Morris1,3
1Division of Perinatal Research, Kolling Institute of Medical Research, Royal North Shore Hospital, University of Sydney, Reserve Rd, St Leonards, NSW 2065, Australia
2School of Medical and Molecular Biosciences, University of Technology Sydney, Sydney, NSW, Australia
3Department of Obstetrics and Gynaecology, Royal North Shore Hospital, St Leonards, NSW, Australia
- *Corresponding Author:
- Sharon McCracken
Division of Perinatal Research
Kolling Institute of Medical Research
Royal North Shore Hospital, Reserve Rd
St Leonards, NSW 2065, Australia
Tel: +61 2 9926 4832
Fax: +61 2 9926 8484
E-mail: Sharon.mccracken@sydney.edu.au
Received date: February 05, 2016; Accepted date: April 13, 2016; Published date: April 18, 2016
Citation: McCracken S, et al. NF-κB Regulation in T-cells in Pregnancy is Mediated via Fas/FasL Interactions: The Signal for which is Derived from Exosomes Present in Maternal Plasma. Reproductiv Immunol Open Acc. 2016, 1:8. doi: 10.4172/2476-1974.100008
Copyright: © 2016 McCracken S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Regulated suppression of maternal Th1 immunity is necessary for normal pregnancy since inappropriate Th1 responses results in increased pregnancy loss and complications including intrauterine growth restriction (IUGR). We have shown that suppression of the p65 subunit of NF-κB in maternal T-cells underlies this change in T-cell responses. This study aimed to determine mechanism(s) that control p65 suppression. Methods and findings: Maternal plasma contained particulate factor’s that suppress p65 and induce T-cell apoptosis in Jurkat T-cells and the factor’s was positive for FasL and TRAIL. Both the Fas activating antibody CH11 and recombinant human TRAIL induced Jurkat apoptosis. Specific Fas activation resulted in p65 suppression which was reversed in the presence of the Fas inactivating antibody ZB4. Despite inducing apoptosis, recombinant TRAIL did not suppress p65 expression. Maternal T-cells expressed increased Fas relative to non-pregnant controls. Fas activation in Jurkats resulted in p65 suppression thus limited IL-2 and IFNï§ transcription in response to PMA/ionomycin stimulation. In contrast, partial knockdown of Fas in Jurkats prevented suppression of p65 in response to CH11, leading to increased IL-2 and IFNï§ production when stimulated with PMA/ionomycin. FasL+ Exosomes isolated from the particulate fraction of maternal plasma specifically induced p65 suppression in Jurkats since suppression was completely reversed with ZB4. In pregnancies complicated with IUGR where Th1 cytokine production is increased, placental expression of FasL was reduced compared to normal controls. Conclusion: Taken together these data suggest that pregnancy derived FasL+ exosomes in maternal plasma regulate p65 levels in circulating T-cells through Fas activation. The expression of Fas on T-cells and FasL on exosomes both dictate the level of p65 suppression and the level of cytokine production in response to stimulation throughout pregnancy. Inappropriate expression of FasL in placental derived exosomes may underlie one mechanism that is abnormal in complications of pregnancy including IUGR.
Keywords
T-cells; Reproductive immunology; Pregnancy; Exosomes
Introduction
Pregnancy requires alterations to maternal T-cell immune responses, such that the fetal allograft is recognized, but not rejected. Upon antigen presentation T-cells can differentiate into immune effector cell types, Th1, Th2, Th17 or regulatory T-cells (Treg). Th1 and Th17 are primarily involved in inflammatory and cellular immunity and protect against intracellular microorganisms and cancer. Th2 cells are involved in humoral immunity and elimination of extracellular pathogens and Tregs promote the induction of tolerance. In pregnancy, successful implantation is dependent on an initial inflammatory response, which is then curtailed to enable pregnancy progression.
Th1 and Th17 cytokines promote acute and chronic allograft rejection, respectively [1,2]. Thus in pregnancy, both Th1 and Th17 responses are suppressed with a concomitant favouring of Th2 immunity and an expansion of Tregs [3,4]. The expansion of Tregs early in pregnancy is essential for protecting the early fetus from rejection since in the mouse depletion of Tregs prior to 10.5 days post coitus (dpc) results in fetal loss [5]. There is insurmountable evidence demonstrating the adverse effect of Th1 and Th17 responses in inducing both early pregnancy loss and intra uterine growth restriction (IUGR) in human and murine pregnancies, and conversely the beneficial effects of Th2 responses in pregnancy success [6,7]. Also clinically the remission of rheumatoid arthritis (RA) in pregnancy [8] is a direct effect of suppressed Th17 immunity since the cytokine IL-17, produced by Th17 cells, plays an essential role in the pathophysiology of rheumatoid arthritis [9]. The mechanisms that regulate these changes in T-cell responses in pregnancy are not fully understood.
NF-κB is key to the regulation of Th1 responses in pregnancy, and in the differentiation of Th17 cells [10]. NF-κB plays a crucial role in Th1 differentiation, clonal expansion and the production of IFNγ [11] as well as in graft rejection in the mouse [12]. The p65:p50 heterodimer is the most common active form of NF-κB and we have shown that expression of p65 is reduced in T-cells throughout pregnancy [13]. This suppression inhibits T-bet expression and ultimately attenuates Th1 cytokine production in response to PMA stimulation [13]. In addition, since Th17 cells require activation of NF-κB for appropriate differentiation, suppression of p65 in pregnancy likely limits the number of functional Th17 cells. Thus, specific regulation of the p65 subunit of NF-κB throughout pregnancy appears to play a central role in maintaining a cytokine environment necessary for normal pregnancy development.
The mechanism by which p65 suppression is regulated in pregnancy is unknown. p65 expression has been shown to be regulated via caspase-mediated degradation in response to activation of Fas. Fas is expressed on activated T-cells and signalling through Fas plays a critical role in the regulation of Tlymphocyte activity, mainly through its role in regulating cell death which is essential for removal of auto-reactive lymphocytes.
Fas is a type I membrane protein of the tumour necrosis factor (TNF)/nerve growth factor (NGF) receptor family [14]. Fas ligand (FasL) is a type II membrane protein that also belongs to the TNF/NGF family. Fas activation of the caspase pathway induces apoptosis. NF-κB is an important mediator of apoptosis through its regulation of various anti-apoptotic genes including Bcl-XL, FLIP and c-IAP1/2 [15]. Cross-linking of Fas specifically targets the p65 subunit of NF-κB for caspase mediated degradation while p50 remains unchanged [16].
Although the factor’s that regulates p65 expression during healthy pregnancy is unknown, we have demonstrated its presence in maternal serum since maternal serum suppresses p65 in human PBMCs from healthy non-pregnant women [17]. The placenta liberates a number of immune modulating factors such as hormones or cytokines [18] and particulate factors including syncytiotrophoblast microparticles STBMs [19] or exosomes that may mediate their effect on NF-κB. Exosomes from both maternal cells and the syncytiotrophoblast are packaged in cytoplasmic multivesicular bodies and subsequently released into the circulation. Exosomes express proteins from their parent cell type and possess biological activity [20] and are considered to be intercellular communicators. In pregnancy exosomes have been shown to be FasL+ and act to induce T-cell apoptosis [21] both placenta [22] and maternal plasma derived exosomes have been shown to possess biological activity which can specifically alter T-cell function.
In this study we test the hypothesis that exosomes derived from maternal plasma are capable of suppressing p65 in T-cells and that this suppressive effect is mediated via Fas activation through FasL+ exosomes. This highlights a potential mechanism that likely plays a role in the regulation of peripheral immunity in pregnancy.
Materials and Methods
Sample cohort
Blood and plasma were collected by informed consent from non-pregnant (NP, n=30) women, not on any form of hormonal contraception, and from pregnant (P, n=30) women in the third trimester of uncomplicated pregnancies (36-40 weeks gestation). P women were recruited from the antenatal clinic at Royal North Shore Hospital (RNSH). Placental samples collected were obtained by informed consent from the same cohort at the time of delivery and from women whose pregnancies were complicated with IUGR <10% (n=14). Ethical approval for this study was granted by the Royal North Shore Hospital Human Research Ethics Committee (1201-046 M).
PBMC isolation, plasma collection and exosome isolation
PBMCs were isolated from blood collected in heparin tubes by standard Ficoll Paque isolation and subsequently used for Tcell isolation or for assessment by Flow cytometry. Plasma was isolated from blood collected in heparin tubes by centrifugation at 400gm for 15 mins. Plasma was stored at -80°C within 1 hr of blood collection for subsequent isolation of exosomes or for use in cell culture. Exosomes were isolated from the plasma of NP and P women as previously described [23]. Diluted plasma was subjected to differential ultracentrifugation and the final 110,000 gm pellet resuspended in 2 mL 20 mM HEPES/2.5 M sucrose and overlaid with 8 x 1.2 mL fractions of sucrose with decreasing concentrations from 2 M to 0.25 M. Samples were spun at 150,000 gm at 4°C overnight. Aliquots (1 ml) of the sucrose gradient were centrifuged in PBS for 75 mins at 110,000gm and re-suspended in PBS and used for analysis by Western blotting, electron microscopy, transmission EM (TEM) or stored at -80°C for use in cell culture. For TEM, exosomes derived from sucrose gradients were fixed in 2% (w/v) paraformaldehyde, loaded on Formwar/carbon-coated EM grids, postfixed in 1% (w/v) glutaraldehyde, and contrasted for analysis by electron microscopy in 1% (w/v) Sodium Silica Tungstate. TEM was performed on a JEOL 1400 TEM.
Cell culture
Primary T-cells were isolated from PBMCs from NP or P women using negative selection according to the manufacturer’s instructions (Dynal, Invitrogen) as previously described [13]. Jurkat T-cells (Sigma) and isolated primary Tcells were maintained at 0.5 x 106 cells/mL in RPMI-1640 (Invitrogen) supplemented with 2 mM L-glutamine (Sigma), 20 U/mL penicillin and streptomycin (Sigma). Serum (either 10% (v/v) fetal calf serum (FCS), 20% (v/v) NP plasma or 20% (v/v) P plasma) or exosomes (150 μg/mL, the concentration previously shown to induce physiological changes in T-cells [23] was added as indicated in figure legends. Cells were maintained in an atmosphere of 5% (v/v) CO2 at 37°C in a humidified incubator.
To assess the role of FasL and TRAIL activation in regulating p65 expression and apoptosis cells were incubated in the presence of the Fas activating antibody CH11 (Millipore) or recombinant human TRAIL (ProSci) at concentrations stated in the figure legends. Analysis of p65 expression levels was assessed by Western blotting. The level of apoptosis was determined using the MitoProbe™ DilC1(5) Assay Kit according to manufacturer’s recommendations (Molecular Probes™).
Western blotting
For western blot, placental tissue (20 mg/mL) was dissociated in RIPA buffer containing 1% (v/v) Antifoam Y-30 Emulsion (Sigma-Aldrich) with the gentleMACS Dissociator (Miltenyi Biotec) according to the manufacturer’s instructions. Jurkat T-cells were lysed at 0.5 x 106/mL and primary T-cells at 1 x 106/mL in RIPA buffer. Exosomes were resuspended in PBS and 10 μg subjected to western blotting. Protein samples were separated on 10% (w/v) SDS-polyacrylamide gels and transferred to nitrocellulose membranes which were blocked as previously described [24]. Using primary antibodies against NF-κB p65 (1:1,000, Santa Cruz), FasL (1:500, Santa Cruz) and TRAIL (2 μg/mL, Abcam) and PD-L1 (0.1 μg/mL, R&D Systems). Blotting with antibodies against β-actin (Sigma) and GAPDH (Santa Cruz) served as loading controls. Antibody binding was detected using HRP-conjugated specific secondary antibodies (Dako) and visualised using enhanced chemiluminescence substrate (ECL+plus Western Blotting Kit, Amersham Pharmacia). Densitometric analysis was performed using ImageQuant TL. The intensity of the protein band of interest was determined using the intensity of the band for GAPDH as a control for protein loading. All OD data is expressed as the % intensity relative to control samples in each separate experiment.
Transfections
siRNAs for Fas and a scrambled control were transfected into Jurkat T-cells using the Amaxa Nucleofector™ as described [3], using the kit specific for Jurkat T-cells. GFP incorporation (supplied) was determined by Flow cytometry to determine transfection efficiency. Cells were cultured post-transfection in RPMI-1640 (Invitrogen) containing 20% (v/v) FCS, 2 mM LGlutamine (Sigma) and 20 U/mL penicillin and streptomycin (Sigma). Media was changed 16 hrs post transfection, and 1.25 μg/mL CH11 added overnight. Cells were subsequently cultured for a further 4 hrs at 37°C +/- 10 ng/mL PMA and 500 ng/mL ionomycin (Sigma).
RNA isolation using Trizol
Treated cells were pelleted via centrifugation and resuspended in Trizol® (Invitrogen, CA, USA.). Total RNA was extracted according to manufacturer’s instructions and dissolved in molecular grade water (Promega Corporation, USA). RNA integrity and concentration were analysed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, Delaware USA). PCR was performed for IFNγ, IL-2 and β2M as previously described [13].
Flow cytometry
Apoptosis was detected in Jurkat T-cells using the MitoProbe™ DilC1(5) Assay Kit. DilC1(5) is a dye that is taken up by cells with active membrane potentials, a loss of DilC1(5) staining indicates cells which have lost membrane potentials and thus viability. The use of Propidium iodide (PI) in conjunction with DilC1(5) identifies those cells that have apoptosed. Staining was carried out according to manufacturer’s recommendations (Molecular Probes™). Briefly 1 mL of cells were incubated with 2 μL DilC1(5) for 30 mins and subsequently pelleted by centrifugation and resuspended in PBS. PI, 1 μL of a 100 μg/mL solution) was added prior to analysis by flow cytometric analysis. Cells which were DilC1(5) negative PI negative were considered cells in early apoptosis and cells DilC1(5) negative PI positive were considered as cells in late apoptosis.
Fas expression was detected on CD3+ T-cells in the lymphocyte gate (determined by size and granularity) from PBMCs from NP and P women using anti-human CD95 (Fas) PE (BD). PBMCs (1 x 106) were pelleted by centrifugation and resuspended in PBS/0.1% (w/v) BSA containing 10 μl of anti- CD95 PE and CD3 FITC (BD). Cells were incubated in the dark at room temperature for 30 mins with gentle agitation, washed in PBS/0.01% (w/v) BSA, and resuspended in 1% (w/v) paraformaldehyde for flow cytometric analysis. CD95+/CD3+ Tcells were identified and the percentages of cells doubly positive were expressed.
All flow cytometry was performed in a BD FACS Vantage SE flow cytometer. Ten thousand events were collected from the CD4+ lymphocyte population of murine splenocytes, the lymphocyte population of primary cells and from Jurkat T-cells. Data analysis was performed using CellQuest Pro Software.
Immunohistochemistry of placenta samples
Placental samples from healthy term pregnancies (n=8) and IUGR (n=14) were fixed overnight in 10% (v/v) neutral buffered formalin, and embedded in paraffin. Sections (5 μm) were exposed to 1% (v/v) H2O2 (Fronine) at RT for 5 min. Staining was performed in the Sequenza system (Thermo Fisher Scientific). Staining for anti-FasL (1:200, Santa Cruz), or isotype control (Dako) was performed at 4°C overnight in Dako antibody diluent, and washed in Dako wash buffer. Antibody binding was detected using the Envision™+ system and HRP labelled anti-Rabbit antibody (DAKO®) and staining visualized using NovRED™ (VECTOR). Slides were counterstained using haematoxylin and Scott’s Blue solution and images captured on a digital Nikon camera.
Statistical analysis
Statistical analysis where appropriate was performed using the Mann Whitney U-test for non-parametric variables and p<0.05 was considered statistically significant. All graphs are expressed as mean ± SEM.
Results
Particulate factors in pregnant plasma alter p65 expression in T-cells
We have previously shown that normal human pregnancy is associated with a suppression of p65 expression in T-cells which acts to regulate T-cell function, specifically through regulating Th1 cytokine production [13]. Human [25] and murine studies [6] and our unpublished data from the CBA/CaH x DBA/2J mouse model, showing increased Th1 cytokine production is associated with increased fetal loss suggests that regulation of cytokine production is required for pregnancy success, thus p65 regulation is an essential component of normal pregnancy progression, yet the mechanism’s that regulate p65 expression are unknown Figure 1.
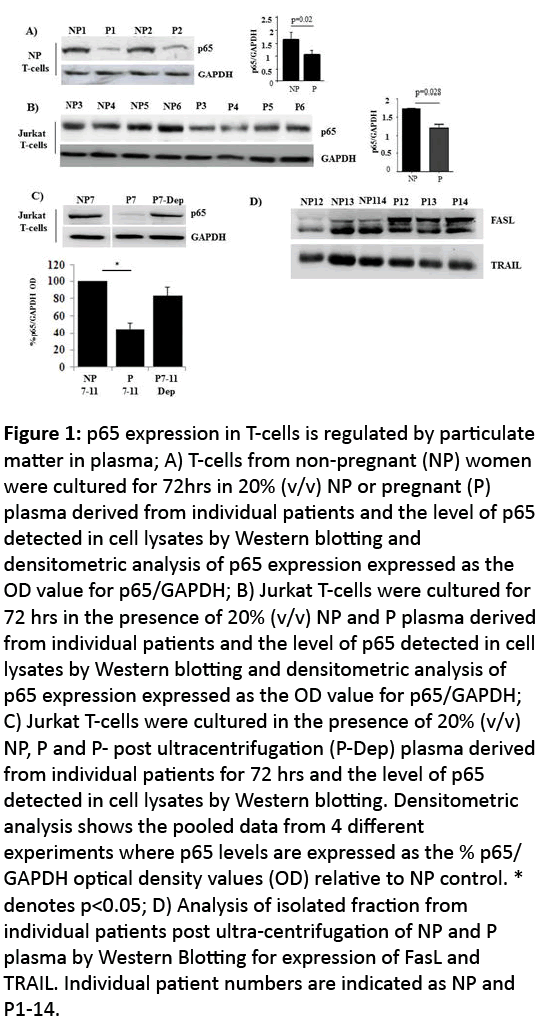
Figure 1: p65 expression in T-cells is regulated by particulate matter in plasma; A) T-cells from non-pregnant (NP) women were cultured for 72hrs in 20% (v/v) NP or pregnant (P) plasma derived from individual patients and the level of p65 detected in cell lysates by Western blotting and densitometric analysis of p65 expression expressed as the OD value for p65/GAPDH; B) Jurkat T-cells were cultured for 72 hrs in the presence of 20% (v/v) NP and P plasma derived from individual patients and the level of p65 detected in cell lysates by Western blotting and densitometric analysis of p65 expression expressed as the OD value for p65/GAPDH; C) Jurkat T-cells were cultured in the presence of 20% (v/v) NP, P and P- post ultracentrifugation (P-Dep) plasma derived from individual patients for 72 hrs and the level of p65 detected in cell lysates by Western blotting. Densitometric analysis shows the pooled data from 4 different experiments where p65 levels are expressed as the % p65/ GAPDH optical density values (OD) relative to NP control. * denotes p<0.05; D) Analysis of isolated fraction from individual patients post ultra-centrifugation of NP and P plasma by Western Blotting for expression of FasL and TRAIL. Individual patient numbers are indicated as NP and P1-14.
We assessed the effect of third trimester maternal plasma on p65 expression in both primary T-cells from NP women (Figure 2A) and in Jurkat T-cells (Figure 2B). Maternal plasma (20% v/v) suppressed p65 expression and the suppressive effect was removed by ultracentrifugation (Figure 2C). Maternal plasma contains many factors that can alter T-cell function including soluble molecules and membrane bound vesicles, since the effect is removed post centrifugation, this suggests the factor responsible for mediating p65 suppression is particulate in matter. We therefore assessed the isolated fraction of plasma post-ultracentrifugation and demonstrated that the fractions from both NP and P plasma samples was positive for FasL and TRAIL (Figure 2D). These two ligands are signalling molecules shown to be expressed by exosomes derived from maternal plasma [20,23] and play a central role in the regulation of apoptosis.
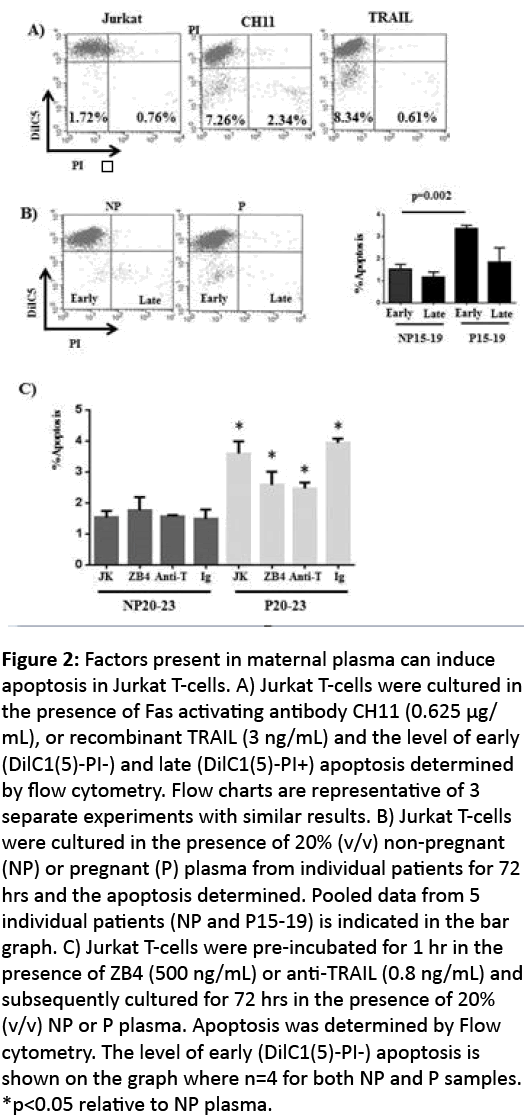
Figure 2: Factors present in maternal plasma can induce apoptosis in Jurkat T-cells. A) Jurkat T-cells were cultured in the presence of Fas activating antibody CH11 (0.625 μg/ mL), or recombinant TRAIL (3 ng/mL) and the level of early (DilC1(5)-PI-) and late (DilC1(5)-PI+) apoptosis determined by flow cytometry. Flow charts are representative of 3 separate experiments with similar results. B) Jurkat T-cells were cultured in the presence of 20% (v/v) non-pregnant (NP) or pregnant (P) plasma from individual patients for 72 hrs and the apoptosis determined. Pooled data from 5 individual patients (NP and P15-19) is indicated in the bar graph. C) Jurkat T-cells were pre-incubated for 1 hr in the presence of ZB4 (500 ng/mL) or anti-TRAIL (0.8 ng/mL) and subsequently cultured for 72 hrs in the presence of 20% (v/v) NP or P plasma. Apoptosis was determined by Flow cytometry. The level of early (DilC1(5)-PI-) apoptosis is shown on the graph where n=4 for both NP and P samples. *p<0.05 relative to NP plasma.
Maternal plasma induces more apoptosis in Jurkat T-cells than NP plasma
NF-κB expression and its subsequent activity is critical for cell survival, thus also plays a central role in the regulation of apoptosis. Maternal plasma contains FasL and TRAIL+ exsosomes. Both of these molecules are capable of inducing apoptosis of Jurkat T-cells (Figure 3A). In response to Fas activating antibody, CH11 and TRAIL the proportion of DilC1(5)-PI-cells which represents early apoptotsis, increased. Since removal of the fraction positive for FasL and TRAIL from maternal plasma results in a loss of p65 suppression in Jurkat T-cell we assessed the effect of NP and P plasma on apoptosis of Jurkat T-cells (Figure 3B). Cells were cultured for 72 hrs in the presence of 20% (v/v) NP and P plasma and apoptosis determined. The proportion of DilC1(5)- PI- cells was significantly higher in cells grown in the presence of P plasma relative to NP plasma, but there was no significant difference in the number of cells that were DilC1(5)- PI+ (late apoptotic events; Figure 3B). We subsequently tested whether the apoptotic signal mediated by P plasma was derived from either Fas or TRAIL activation. Inhibition of either Fas or TRAIL only partially reversed the early apoptosis induced by P plasma. Neither Fas nor TRAIL inactivation affected cells grown in NP plasma (Figure 3C).
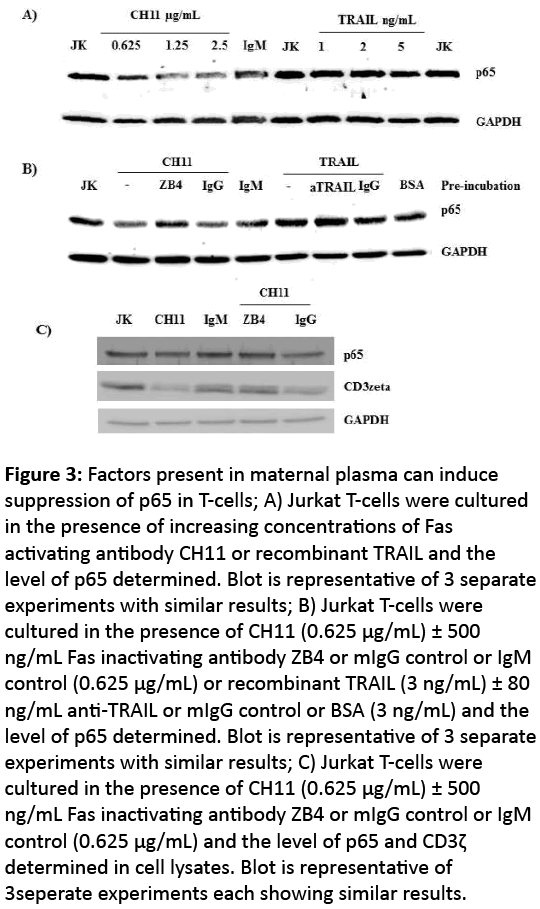
Figure 3: Factors present in maternal plasma can induce suppression of p65 in T-cells; A) Jurkat T-cells were cultured in the presence of increasing concentrations of Fas activating antibody CH11 or recombinant TRAIL and the level of p65 determined. Blot is representative of 3 separate experiments with similar results; B) Jurkat T-cells were cultured in the presence of CH11 (0.625 μg/mL) ± 500 ng/mL Fas inactivating antibody ZB4 or mIgG control or IgM control (0.625 μg/mL) or recombinant TRAIL (3 ng/mL) ± 80 ng/mL anti-TRAIL or mIgG control or BSA (3 ng/mL) and the level of p65 determined. Blot is representative of 3 separate experiments with similar results; C) Jurkat T-cells were cultured in the presence of CH11 (0.625 μg/mL) ± 500 ng/mL Fas inactivating antibody ZB4 or mIgG control or IgM control (0.625 μg/mL) and the level of p65 and CD3ζ determined in cell lysates. Blot is representative of 3seperate experiments each showing similar results.
Fas activation results in reduced p65 expression in Jurkat T-cells
The induction of apoptosis in Jurkat T-cells in response to both Fas activation and TRAIL, suggests that since p65 plays a crucial role in cell survival either one or both of these molecules is involved in regulating the suppression of p65 in Tcells. In response to increasing concentrations of the Fas activating antibody CH11, p65 levels were diminished in Jurkat T-cell lysates (Figure 4A) and the suppression was reversed using the Fas inactivating antibody ZB4 (Figure 4B). However despite inducing apoptosis in Jurkat T-cells, incubation with increasing concentrations of recombinant TRAIL had no effect on p65 levels (Figure 4A). Suggesting Fas activation underpins the control of p65 in T-cells.
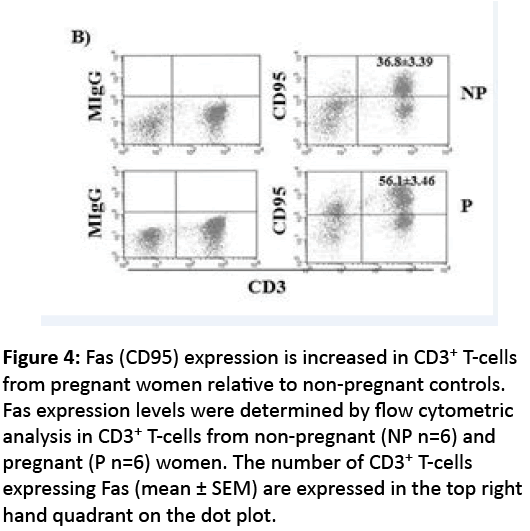
Figure 4: Fas (CD95) expression is increased in CD3+ T-cells from pregnant women relative to non-pregnant controls. Fas expression levels were determined by flow cytometric analysis in CD3+ T-cells from non-pregnant (NP n=6) and pregnant (P n=6) women. The number of CD3+ T-cells expressing Fas (mean ± SEM) are expressed in the top right hand quadrant on the dot plot.
Fully functioning T-cells require appropriate expression of p65 and CD3ζ, a moiety required for TCR activation, thus we assessed the effect of Fas activation on CD3ζ as well as p65 expression in Jurkat T-cells. Fas activation in Jurkat T-cells using CH11 induced CD3ζ as well as p65 suppression and this was completely reversed with the Fas inactivating antibody ZB4 (Figure 4C).
Fas expression on T-cells is required to enable p65 suppression in response to Fas activation and reduced Th1 cytokine production in response to PMA stimulation
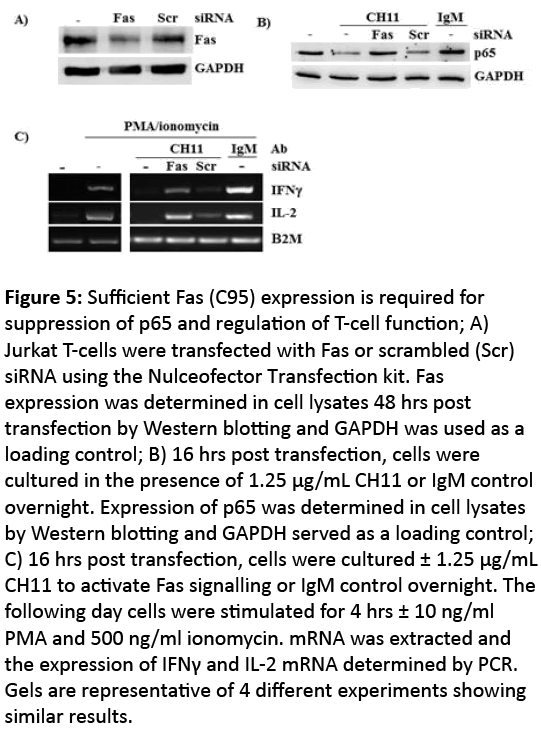
Since Fas activation regulates T-cell function and potentially underlies the regulation of p65 expression in T-cells in pregnancy, we assessed Fas expression in T-cells in pregnancy and showed by Flow cytometry that Fas expression was significantly increased in CD3+ T-cells from P women relative to NP controls (Figure 5). To determine whether Fas activation was fundamentally required for regulating p65 degradation in T-cells we knocked down Fas on Jurkat T-cells using siRNA. Transfection efficiency was tested using GFP and 70% of cells were routinely GFP+ (data not shown). Figure 6A shows approximately 40% knockdown of Fas expression 48 hrs post transfection. 16 hrs after transfection, cells were stimulated with CH11 (overnight). Down regulation of Fas expression resulted in an inability of Jurkat T-cells to suppress p65 expression in response to CH11 (Figure 6B). Conversely, untransfected cells, or those transfected with scrambled siRNA showed suppression of p65 in response to CH11 (Figure 6C).
Figure 5: Sufficient Fas (C95) expression is required for suppression of p65 and regulation of T-cell function; A) Jurkat T-cells were transfected with Fas or scrambled (Scr) siRNA using the Nulceofector Transfection kit. Fas expression was determined in cell lysates 48 hrs post transfection by Western blotting and GAPDH was used as a loading control; B) 16 hrs post transfection, cells were cultured in the presence of 1.25 μg/mL CH11 or IgM control overnight. Expression of p65 was determined in cell lysates by Western blotting and GAPDH served as a loading control; C) 16 hrs post transfection, cells were cultured ± 1.25 μg/mL CH11 to activate Fas signalling or IgM control overnight. The following day cells were stimulated for 4 hrs ± 10 ng/ml PMA and 500 ng/ml ionomycin. mRNA was extracted and the expression of IFNγ and IL-2 mRNA determined by PCR. Gels are representative of 4 different experiments showing similar results.
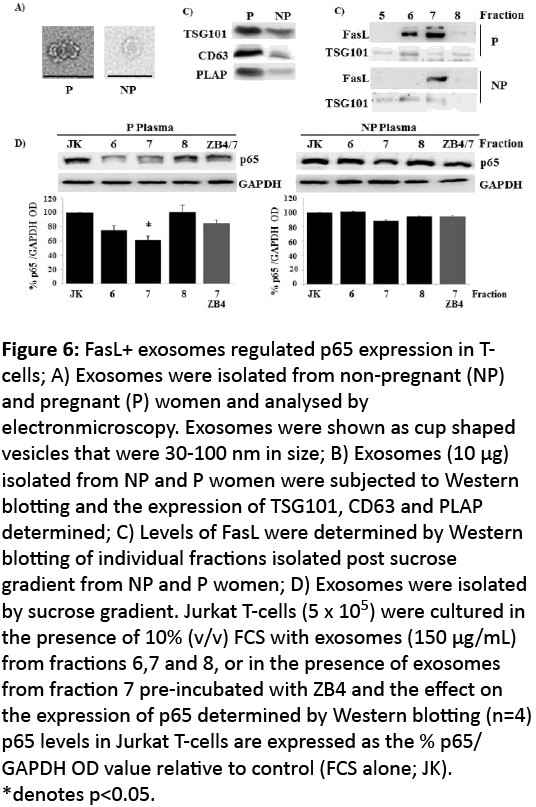
Figure 6: FasL+ exosomes regulated p65 expression in Tcells; A) Exosomes were isolated from non-pregnant (NP) and pregnant (P) women and analysed by electronmicroscopy. Exosomes were shown as cup shaped vesicles that were 30-100 nm in size; B) Exosomes (10 μg) isolated from NP and P women were subjected to Western blotting and the expression of TSG101, CD63 and PLAP determined; C) Levels of FasL were determined by Western blotting of individual fractions isolated post sucrose gradient from NP and P women; D) Exosomes were isolated by sucrose gradient. Jurkat T-cells (5 x 105) were cultured in the presence of 10% (v/v) FCS with exosomes (150 μg/mL) from fractions 6,7 and 8, or in the presence of exosomes from fraction 7 pre-incubated with ZB4 and the effect on the expression of p65 determined by Western blotting (n=4) p65 levels in Jurkat T-cells are expressed as the % p65/ GAPDH OD value relative to control (FCS alone; JK). *denotes p<0.05.
In pregnancy the reduction of p65 in CD3+ T-cells renders these cells unable to induce Th1 cytokines in response to PMA/ionomycin [13]. We tested whether suppression of p65 using CH11 was sufficient to affect the ability of these cells to produce the Th1 cytokines IFNγ and IL-2 in response to PMA/ ionomycin. Jurkat T-cells stimulated for 4 hrs with PMA/ ionomycin showed increased levels of both IFNγ and IL-2 mRNA (Figure 6C); in contrast CH11 activation prior to PMA/ ionomycin stimulation resulted in an inability to induce both IFNγ and IL-2 (Figure 6C). Cells transfected with Fas siRNA had reduced Fas expression levels thus unable to alter p65 levels in response to CH11 (Figure 6B). As such subsequent stimulation with PMA/ionomycin resulted in an increased production of IFNγ and IL-2 mRNA relative to both untransfected and scrambled siRNA transfected Jurkat T-cells (Figure 6C).
FasL+ exosomes induce p65 suppression in Tcells
Our data suggest Fas activation by a particulate factor(s) present in maternal plasma mediates p65 suppression and subsequently reduces the ability to produce Th1 cytokines in Tcells. The syncytiotrophoblast layer of the placenta is FasL+ [21] and thus a potential source of the FasL+ exosomes that are present in maternal plasma. We assessed whether FasL+ exosomes are the source of p65 suppression in normal pregnancies. We isolated exosomes from both NP and P plasma by differential ultracentrifugation. Exosomes were identified by electron microscopy as cup shaped vesicles 30 nm -100 nm in diameter (Figure 7A) and were characterised biochemically by their expression of CD63 and TSG101 (Figure 7B). In addition, only exosomes from P plasma were PLAP positive (Figure 7B) demonstrating a placental origin for at least a proportion of the isolated exosomes.
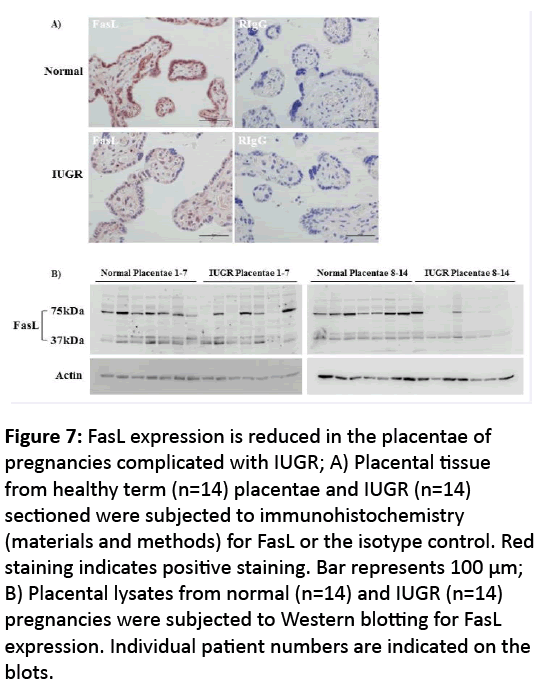
Figure 7: FasL expression is reduced in the placentae of pregnancies complicated with IUGR; A) Placental tissue from healthy term (n=14) placentae and IUGR (n=14) sectioned were subjected to immunohistochemistry (materials and methods) for FasL or the isotype control. Red staining indicates positive staining. Bar represents 100 μm; B) Placental lysates from normal (n=14) and IUGR (n=14) pregnancies were subjected to Western blotting for FasL expression. Individual patient numbers are indicated on the blots.
Exosomes were further purified on a sucrose gradient and individual fractions were shown to be TSG101 positive (Figure 7C). In P samples, 2 fractions were also consistently positive for FasL while only 1 fraction was FasL positive from NP samples (Figure 7C). Jurkat T-cells were incubated in the presence of isolated exosomes. Fractions that were FasL+ from P plasma induced significant suppression of p65 (Figure 7D), which was reversed when cells were pre-incubated with ZB4. Exosomes from NP plasma did not affect p65 expression in Jurkat T-cells.
Our data suggest that FasL+ exosomes that are derived at least in part from the placenta regulate maternal T-cell function by controlling p65 expression and thus cytokine production. Abnormal immune regulation in pregnancy has been shown to be associated with multiple pregnancy complications including IUGR. We assessed the expression of FasL in the placenta from pregnancies complicated with IUGR and showed that by immunohistochemistry, expression of FasL was reduced in placentae from pregnancies complicated with IUGR relative to normal controls. In addition, only placentae from normal uncomplicated pregnancies consistently (14/14) expressed the high 75 kDa FasL band compared to 5/14 placentae from IUGR pregnancies. This suggests exosomes present in the maternal plasma that are derived from the placenta may be deficient in FasL expression and therefore not alter T-cell function accordingly.
Discussion
Changes in the maternal immune system are necessary for the success of implantation as well as the development and maintenance of human pregnancies. Our understanding of the mechanisms that govern these alterations is incomplete. In this study we describe a potential role for pregnancy specific exosomes in regulating T-cell responses. We have previously shown that the suppression of NF-κB (p65) in T-cells in normal pregnancy is a mechanism by which favourable T-cell responses are maintained [13]. We now show for the first time that this pregnancy mediated suppression of p65 is regulated by pregnancy specific signalling through the release of FasL bearing exosomes targeting maternal T-cells expressing Fas.
The deleterious effect of both Th1 and Th17 cytokine production during pregnancy is well recognised. We have previously shown a hierarchy in transcriptional control that regulates Th1 cytokine production from T-cells in pregnancy, with NF-κB activity an essential requirement for the transactivation of T-bet, the master regulator of Th1 immunity [13]. Similarly, complete differentiation of the Th17 phenotype is dependent on appropriate NF-κB activity [10]. We demonstrated from the late first trimester of pregnancy through to term, that the p65 dimer of NF-κB is suppressed and that this regulates the severity of Th1 responses [13] and potentially the number of Th17 cells throughout pregnancy. Published [6] and our unpublished data from the CBA/CaH x DBA/2J murine cross showing increased Th1 cytokine production being consistent with fetal loss, suggest that appropriate cytokine regulation and therefore p65 control is essential for normal pregnancy success. Despite this, the mechanisms that regulate p65 suppression in pregnancy are unknown.
Maternal plasma and serum contain many factors known to influence the immune system. We have shown that maternal plasma suppresses p65 expression in PBMCs [26] and in isolated T-cells from NP women and that the factor that regulates p65 expression is particulate in nature as ultracentrifugation of maternal plasma removes the suppressive effect.
The pellets isolated from plasma by ultracentrifugation are crude fractions which contain an as yet undefined collection of microvesicles which would include apoptotic membranes and exosomes and other particulate material which may have an effect on T-cell function. Irrespective of the nature of the particulate material, removal was sufficient to eliminate the suppressive effect of maternal plasma on p65 suppression in Jurkats. Phenotypic assessment of the pellet isolated from both NP and P plasma demonstrated positivity for FasL and TRAIL, both of which are expressed on exosomes and individually known for their role in inducing apoptosis [27,28]. In addition to regulating Th1 cytokine production, NF-κB is a key regulator of apoptosis due to the transcription control of cell survival proteins which include Bcl-2, FLIP and BclXL [29]. RelA (p65) knockout mice are embryonic lethal, due to massive degeneration of the liver through apoptosis [30]. We demonstrated that maternal plasma, could induce significantly more apoptosis of Jurkat T-cells than non-pregnant controls, and that the apoptosis was partially reversed by blocking both Fas activation and by inhibiting TRAIL activity in cells cultured in the presence of P plasma, but not NP plasma. Although FasL and TRAIL were detectable in the fraction isolated from NP plasma, the inability to affect apoptosis suggests that these proteins isolated from P plasma are packaged (we would suggest in exosomes) in a way that allows specific targeting to T-cells, though the mechanisms are yet to be determined. The partial inhibition of P plasma induced apoptosis using Fas inhibition and anti-TRAIL antibodies suggests that both pathways are involved in the regulation of apoptosis during pregnancy. This is consistent with a low correlation between the level of FasL on exosomes derived from maternal plasma and the induction of apoptosis in Jurkat T-cells [23] and with reports demonstrating apoptosis of Jurkat T-cells and activated PBMCs in response to placental specific FasL+ and TRAIL+ exosomes derived from either placental cultures or from maternal plasma [20,21].
Despite recombinant TRAIL inducing apoptosis in Jurkat Tcells, it failed to alter p65 expression levels. In contrast, Fas activation not only induced apoptosis, but resulted in the suppression of p65 expression. Stenqvist et al. [21] recently demonstrated that exosomes derived from placental tissue expressed either FasL or TRAIL but did not express both on the same exosome. It seems more than appropriate that at least two mechanisms that control T-cell function by regulating Tcell activation-induced cell death exist in pregnancy and that both would likely contribute towards the immune privilege of the fetus. Indeed despite Vacchio and Hodes [31] demonstrating that fetal FasL expression induced CD8+ T-cell tolerance to the fetal antigen H-Y during pregnancy, and that the loss of placental FasL is associated with increased fetal loss and thus small litter sizes [32], Chaouat and Clark suggested that apoptosis of T-cells in allopregnancies were mediated via mechanisms other than Fas+/FasL+ [33]. Those alternate mechanisms would no doubt include TRAIL/TRAIL receptors (DR4 and DR5) and PD-L1/PD-1, another immune modulating moiety known to be expressed on exosomes [34].
Although multiple mechanisms play a role in maintaining fetal tolerance in pregnancy there is increasing evidence demonstrating that exosomes isolated from first [35] and third trimester placental tissue [21] and third trimester maternal plasma [23] can alter T-cell function specifically through activation of Fas. Our data shows that the level of Fas expression is a limiting factor in T-cell responsiveness since partial knock down of Fas in Jurkat T-cells renders them unresponsive to Fas mediated p65 suppression. Conversely, the amount of FasL expression on exosomes derived from pregnant serum and plasma has been shown to correlate with their ability to induce CD3ζ suppression in Jurkat T-cells [20,36] which is consistent with our observation that specific activation of Fas results in reduced CD3ζ as well as p65 expression in Jurkat T-cells. A conclusive role for FasL+ exosomes as mediators regulating p65 expression in T-cells comes from our observation that blocking Fas activation using the blocking antibody ZB4 only partially reverses P plasma mediated apoptosis, but completely reverses the p65 suppression in response to plasma derived exosomes. In contrast although a correlation between FasL+ exosomes and CD3ζ suppression exists, blocking with ZB4 only partially reverses the suppression of CD3ζ in response to exosomes [20,23].
T-cell tolerance is associated with an inability to induce IL-2 production in response to antigen re-stimulation. After TCR engagement, CD3ζ phosphorylation initiates a signal transduction pathway that leads to T-cell activation and the induction of IL-2 production [37]. Activation of NF-κB also results in IL-2 production. Taylor et al. showed that in addition to suppressing CD3ζ expression plasma derived placentalexosomes were capable of suppressing IL-2 production [20]. We have shown that p65 is required for IL-2 production [13] and conversely that inhibition of p65 expression suppresses IL-2 production in response to PMA activation. Thus together, the control of these two molecules that play a fundamental role in regulating T-cell function may represent the induction of T-cell energy in response to plasma derived exosomes that is necessary for pregnancy progression.
Both Taylor et al. and our group have shown that Fas activation appears to underlie the regulation of CD3ζ (20) and p65 and that these signals come from exosomes present in maternal plasma. The exact nature of exosome-T-cell interactions are as yet not fully understood, but increasing numbers of molecules that could specifically target exosomes to T-cells are being identified including the expression of MHC Class I and II molecules in pregnancy specific exosomes [20]. Indeed despite that fact that we were able to isolate FasL+ and TRAIL+ particles from the plasma of non-pregnant and pregnant women, only those from pregnant women were able to induce a significant reduction in p65 levels in Jurkat T-cells. Similarly, exosomes isolated from NP women were less able to alter CD3ζ and IL-2 levels than those isolated from P women [20]. This suggests that targeting moieties exist in pregnancy derived exosomes that direct their effect specifically to maternal T-cells. Whether or not Fas activation results in the CD3ζ directly or indirectly is unknown at present. In contrast, Fas activation has been shown to directly regulate p65 expression. Fas activation results in a caspase cascade that culminates in the degradation of p65 but does not affect p50 expression, the same scenario we see in T-cells in pregnancy where despite a reduction in p65 protein, p50 protein levels remain stable [38]. This together with our current data suggests FasL expression on exosomes underlies the regulation of p65 in T-cells in pregnancy.
The source of exosomes isolated in this study were from maternal plasma and are therefore potentially of fetal and/or maternal origin, we and others have shown recently that FasL is detectable in the syncytiotrophoblast of third trimester human placentae [21], the area of the placenta that is in direct contact with maternal blood, and thus a likely source of the exosomes we isolated from the maternal plasma. The expression of FasL at the maternal fetal interphase has long been thought to promote immune suppression and provide the fetus with the immune privilege necessary for pregnancy success, since at the site of implantation apoptotic T-cells are consistently detected [39]. The notion of the expression of FasL at the maternal/fetal interface conferring immune privilege for the fetus has however been challenged by the observation from in vivo data showing that membrane bound FasL may actually promote graft and tumour rejection rather than survival. Membrane FasL-expression can induce neutrophil infiltration and rejection of transplanted beta islet cells in allogenic mice [40,41] and FasL+ colon cancer cells are rejected rather than protected when injected subcutaneously in mice again due to mass neutrophil infiltration [42]. Using electron microscopy, Stenqvist et al. have shown clearly that FasL is not expressed on the apical surface of the syncytiotrophoblast, but rather is expressed internally in endosomal compartments of the syncytiotrophoblast and subsequently exposed when packaged into exosomes [21]. Similarly Abrahams showed that first trimester trophoblast cells lacked membrane-associated FasL, but contained bioactive FasL packaged in cytoplasmic vesicles [35]. Both groups suggest that the packaging of FasL in these vesicles eliminates the possibility that expression of membranous FasL by the placenta would induce neutrophil infiltration which may subsequently result in rejection rather than protection of the placenta. In addition, the packaging of FasL inside or on exosomal membranes enables the delivery of an apoptotic inducing signal to activated maternal immune cells in the peripheral circulation, thus contributing to the protection of the fetal-placental unit.
The number of FasL+ exosomes in plasma increases throughout pregnancy [20,23]. Activated T-cells express Fas and we and others [43] have shown that Fas expression is increased in T-cells in pregnant women relative to nonpregnant women, possibly due to exposure of T-cells to paternal antigens throughout pregnancy. The increased expression of both Fas and FasL in pregnancy highlights the potential for this pathway to play a crucial role in peripheral regulation of maternal immunity, which our data suggests is at least partially regulated by the level of p65 expression.
Pregnancy complications including recurrent pregnancy loss and IUGR are thought to have an underlying maternal immunological pathophysiology. In patients suffering from implantation failure and recurrent pregnancy loss, the production of Th1 cytokines from isolated T-cells is increased relative to patients with normal pregnancies [44,45], our data from the CBA/CaH x DBA/2J confirms this. In addition, in the third trimester of pregnancy T-cells from patients with pregnancies complicated with IUGR express greater levels of IFNγ than those from normal pregnant controls [7]. We have shown that appropriate expression of p65 is essential for stimulation induced production of Th1 cytokines [13], suggesting that in these pregnancy pathologies ineffective alteration of p65 expression in maternal T-cells may underlie their progression. Our current data showing reduced expression of FasL on the placentae from pregnancies complicated with IUGR would suggest that exosomes derived from these pathological placentae lack appropriate Fas/FasL signalling and are unable to alter p65 expression in T-cells. Consistent with this, Taylor et al. [20] demonstrated that exosomes isolated from the plasma of women whose pregnancies were complicated with pre-term delivery were less effective at inhibiting IL-2 production in response to stimulation relative to exosomes isolated from normal pregnancies. It is pertinent therefore to determine whether exosomes derived from pregnancies complicated with IUGR are equally less able to alter p65 expression.
Thus we can conclude that P plasma contains FasL+ and TRAIL+ exosomes (a proportion of which are placental derived). FasL+ exosomes can activate Fas bound to maternal T-cells and induce p65 suppression, leading to a reduction in Th1 cytokine production. TRAIL can induce T-cell apoptosis but it does not suppress p65 expression. Altogether our data suggest that the expression of immune modulating molecules on the surface of exosomes has the potential to modulate maternal immunity that likely plays a role in the regulation of peripheral immunity in pregnancy.
Funding
Funding for this study was provided by the NHMRC and Ramsay Health Care, Royal North Shore Hospital, St Leonards, Sydney, Australia.
References
- Kaminski ER, Kaminski A, Bending MR, Chang R, Heads A, et al. (1995) In vitro cytokine profiles and their relevance to rejection following renal transplantation.Transplantation 60: 703-706.
- Subramanian V1, Mohanakumar T (2012) Chronic rejection: a significant role for Th17-mediated autoimmune responses to self-antigens. Expert Rev ClinImmunol 8: 663-672.
- McCracken SA, Hadfield K, Rahimi Z, Gallery ED, Morris JM (2007) NF-kappaB-regulated suppression of T-bet in T cells represses Th1 immune responses in pregnancy. Eur J Immunol 37: 1386-1396.
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT (2004) Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112: 38-43.
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, et al. (2010) Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J ReprodImmunol 85: 121-129.
- Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, et al. (1990) Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J ReprodFertil 89: 447-458.
- Raghupathy R, Al-Azemi M, Azizieh F (2012) Intrauterine growth restriction: cytokine profiles of trophoblast antigen-stimulated maternal lymphocytes. ClinDevImmunol 2012: 734865.
- Ostensen M (1999) Sex hormones and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Ann N Y AcadSci 876: 131-143.
- Shen H, Goodall JC, Hill Gaston JS (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum 60: 1647-1656.
- Molinero LL, Cubre A, Mora-Solano C, Wang Y, Alegre ML (2012) T cell receptor/CARMA1/NF-κB signaling controls T-helper (Th) 17 differentiation. ProcNatlAcadSci U S A 109: 18529-18534.
- Corn RA, Aronica MA, Zhang F, Tong Y, Stanley SA, et al. (2003) T cell-intrinsic requirement for NF-kappa B induction in postdifferentiation IFN-gamma production and clonal expansion in a Th1 response. J Immunol 171: 1816-1824.
- Finn PW, Stone JR, Boothby MR, Perkins DL (2001) Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. J Immunol 167: 5994-6001.
- Hadfield KA, McCracken SA, Ashton AW, Nguyen TG, Morris JM (2011) Regulated suppression of NF-kappaB throughout pregnancy maintains a favourable cytokine environment necessary for pregnancy success. J ReprodImmunol.
- Lee HO, Ferguson TA (2003) Biology of FasL. Cytokine Growth Factor Rev 14: 325-335.
- Kulms D, Schwarz T (2006) NF-kappaB and cytokines. VitamHorm 74: 283-300.
- Wong HK, Tsokos GC (2006) Fas (CD95) ligation inhibits activation of NF-kappa B by targeting p65-Rel A in a caspase-dependent manner. ClinImmunol 121: 47-53.
- McCracken SA, Drury CL, Lee HS, Morris JM (2003) Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J ReprodImmunol 58: 27-47.
- 18 Kalantaridou SN, Zoumakis E, Makrigiannakis A, Godoy H, Chrousos GP (2007) The role of corticotropin-releasing hormone in blastocyst implantation and early fetalimmunotolerance. Hormone & Metabolism Research 39: 474-477.
- Southcombe J, Tannetta D, Redman C, Sargent I (2011) The immunomodulatory role of syncytiotrophoblastmicrovesicles. PLoS One 6: e20245.
- Taylor DD, Akyol S, Gercel-Taylor C (2006) Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 176: 1534-1542.
- Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L (2013) Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J Immunol 191: 5515-5523.
- Nieuwland R, van der Post JA, Lok CA, Kenter G, Sturk A (2010) Microparticles and exosomes in gynecologicneoplasias. SeminThrombHemost 36: 925-929.
- Sabapatha A, Gercel-Taylor C, Taylor DD (2006) Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J ReprodImmunol 56: 345-355.
- McCracken SA, Gallery E, Morris JM (2004) Pregnancy-specific down-regulation of NF-kappa B expression in T cells in humans is essential for the maintenance of the cytokine profile required for pregnancy success. J Immunol 172: 4583-4591.
- Raghupathy R (1997) Th1-type immunity is incompatible with successful pregnancy. Immunol Today 18: 478-482.
- McCracken SA, Drury CL, Lee HS, Morris JM (2003) Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J ReprodImmunol 58: 27-47.
- Wajant H (2003) Death receptors. Essays Biochem 39: 53-71.
- Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, et al. (2005) Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol 174: 3408-3415.
- Kulms D, Schwarz T (2006) NF-kappaB and cytokines. VitamHorm 74: 283-300.
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376: 167-170.
- Vacchio MS, Hodes RJ (2005) Fetal expression of Fas ligand is necessary and sufficient for induction of CD8 T cell tolerance to the fetal antigen H-Y during pregnancy. J Immunol 174: 4657-4661.
- Hunt JS, Vassmer D, Ferguson TA, Miller L (1997) Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol 158: 4122-4128.
- Chaouat G, Clark DA (2001) FAS/FAS ligand interaction at the placental interface is not required for the success of allogeneic pregnancy in anti-paternal MHC preimmunized mice. Am J ReprodImmunol 45: 108-115.
- Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, et al. (2012) Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 33: 982-990.
- Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G (2004) First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod 10: 55-63.
- Taylor DD, Sullivan SA, Eblen AC, Gercel-Taylor C (2002) Modulation of T-cell CD3-zeta chain expression during normal pregnancy. J ReprodImmunol 54: 15-31.
- Samelson LE (2002) Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol 20: 371-394.
- Wong HK, Tsokos GC (2006) Fas (CD95) ligation inhibits activation of NF-kappa B by targeting p65-Rel A in a caspase-dependent manner. ClinImmunol 121: 47-53.
- Mor G, Gutierrez LS, Eliza M, Kahyaoglu F, Arici A (1998) Fas-fas ligand system-induced apoptosis in human placenta and gestational trophoblastic disease. Am J ReprodImmunol 40: 89-94.
- Allison J, Georgiou HM, Strasser A, Vaux DL (1997) Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. ProcNatlAcadSci U S A 94: 3943-3947.
- Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, et al. (1997) Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med 3: 738-743.
- Chen JJ, Sun Y, Nabel GJ (1998) Regulation of the proinflammatory effects of Fas ligand (CD95L). Science 282: 1714-1717.
- Reinhard G, Noll A, Schlebusch H, Mallmann P, Ruecker AV (1998) Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. BiochemBiophys Res Commun 245: 933-938.
- Calleja-Agius J, Brincat MP (2008) Recurrent miscarriages: What is the role of cytokines? GynecolEndocrinol 24: 663-668.
- Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas E, Mangubat CP, et al. (2003) Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod 18: 767-773.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences